Schiff base transition metal complexes for N-Arylation of heterocycles under mild conditions
Abstract
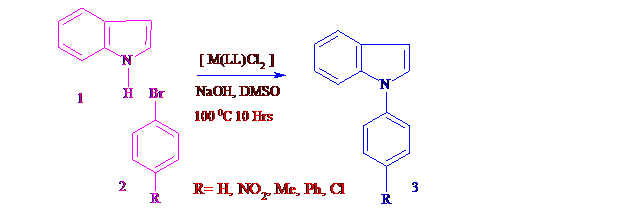
A series of schiff base transition metal complexes [M(2L)X2] where M= Co (II), Ni(II), Cu(II) and Zn(II), X= Cl and L= 2-Phenyl 3- benzoylamino 1,2 -Dihydro Quinazolin-4(3H)-one (PBADQ) were synthesized by the reaction of Schiff base PBADQ and transition metal salts CoCl2.6H2O, NiCl2.6H2O, CuCl2.2H2O and ZnCl2. The Schiff base ligand L and complexes 1-4 were characterized by C, H, N elemental analysis, IR, NMR spectral studies. The catalytic activity of these complexes was tested for N-Arylation of Indole and Benzoimidazole and observed that all the complexes worked as the efficient catalysts for the N-Arylation of Indole and Benzoimidazole with Bromobenzene and its derivatives at moderate reaction conditions in DMSO.
Â
Â
                                 Â
Â
Â
Keywords:Â Quinazolinone Schiff base, transition metal complexes, N-Arylation
Â
Corresponding author: Tel.: +91 2343221632; fax: +91 2343221632.
                                      E-mail: gaikwadga@rediffmail.com
Keywords
Full Text:
PDFReferences
F. Monnier and M. Taillefer, “Catalytic C-C C-N, and C-O Ullmann-Type Coupling Reactions,†Angewandte Chemie International Edition, 2009, 48, 6954-6971.
D. S. Surry and S. L. Buchwald, “DialkylbiarylPhosphines in Pd-Catalyzed Amination: A User’s Guide,†Chemical Science, 2011, 2, 27-50.
D. Maiti, B. P. Fors, J. L. Henderson, Y. Nakamura and S. L. Buchwald, “Palladium Catalyzed Coupling of Functionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases,†Chemical Science, 2011, 2, 57-68.
S. L. Buchwald, A. Klapars, J. C. Antilla, G. E. Job, M. Wolter, F. Y. Kwong, G. Nordmann and E. J. Hennessy, “Copper Catalyzed Formation of Carbon Heteroatom and Carbon-Carbon Bonds,†US 2001 0286286-WO02/085838.
A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, “A General And Efficient Copper Catalyst for the Amidation of Aryl Halides and the N-Arylation of Nitrogen Heterocycle,†Journal of the American Chemical Society, 2001, 123, 7727-7729.
M.Taillefer, H.-J. Cristau, P. P. Cellier, J.-F. Spindler and A. Ouali, “Method for Forming a Carbon-Carbon or Carbon-Heteroatom Linkage,†Fr 2001, 16547-WO035 3225.
Klapars, X. Huang and S. L. Buchwald, “A General and Efficient Copper Catalyst for the Amidation of Aryl Halides,†Journal of the American Chemical Society, 2002, 124, 7421-7428.
Z. Lu and R. Twieg, “Copper-Catalyzed Aryl Amination in AqueousMedia with 2- DimethylaminoethanolLigand,†Tetrahedron Lettersers, 2005, 46, 2997-3001.
H. Rao, H. Fu, Y. Jiang and Y. Zhao, “Copper-Catalyzed Arylation of Amines Using Diphenyl Pyrrolidine-2-Phosphonate as the New Ligand,†The Journal of Organic Chemistry, 2005, 70, 8107- 8109.
H. Zhang, Q. Cai and D. Ma, “Amino Acid Promoted CuI-Catalyzed C-N Bond Formation between Aryl Hal-ides and Amines or N-Containing Heterocycles,†The Journal of Organic Chemistry, 2005, 70, 5164-5173.
Y. Chen and H. Chen, “1,1,1-Tris(hydroxymethyl)ethane as A New, Efficient, and Versatile Tripod Ligand for Copper-Catalyzed Cross-Coupling Reactions of Aryl Iodides with Amides, Thiols, and Phenols,†Organic Letters, 2006, 8, 5609-5612.
M. Yang and F. Liu, “Diamine Ligands in Copper-Ca- talyzed Reactions, An Ullmann Coupling of Aryl Iodides and Amines Using An Air-Stable Diazaphospholane Ligand,†The Journal of Organic Chemistry, 2007,72, 8969-8971.
P. Suresh and K. Pitchumani, “Per-6-amino-β-cyclodex- trin as An Efficient Supramolecular Ligand and Host for Cu(I)-Catalyzed N-Arylation of Imidazole with Aryl Bromides,†The Journal of Organic Chemistry, 2008, 73, 9121-9124.
D. Wang and K. Ding, “2-Pyridinyl β-Ketones as New Ligands for Room Temperature CuICatalysed C-N Coupling Reactions,†Chemical Communications, 2009, 1891-1893.
H. Zhao, H. Fu and R. Qiao, “Copper Catalyzed Direct Amination of Ortho Functionalized Haloarenes with Sodium Azide as the Amino Source,†The Journal of Or- ganic Chemistry, 2010, 75, 3311-3316.
K. G. Thakur, K. S. Srinivas, K. Chiranjeevi and G. Sekar, “D-Glucosamine as An Efficient Ligand for the Copper Catalyzed Selective Synthesis of Anilines from Aryl Ha lides and NaN3,†Green Chemistry, 2011, 13, 2326-2329.
Wang, F. Zhang, D. Kuang, J. Yu and J. Li, “A Highly Efficient Cu Catalyst System for N-Arylation of Azoles in Water,†Green Chemistry, 2012, 14, 1268-1271.
Z. Q. Wu, Z. Q. Jiang, D. Wu, H. F. Xiang and X. G. Zhou, “A Simple and Efficient Catalytic System for Coupling Aryl Halides with Aqueous Ammonia in Water,†European Journal of Organic Chemistry, 2010, 1854-1857.
Z. Q. Wu, L. Zhou, Z. Q. Jiang, D. Wu, Z. K. Li and X. G. Zhou, “Sulfonato-Cu(salen) Complex Catalyzed N-Arylation of Aliphatic Amines with Aryl Halides in Water,†European Journal of Organic Chemistry, 2010, 4971-4975.
Y. Wang, Z. Wu, L. X. Wang, Z. K. Li and X. G. Zhou, “A Simple and Efficient Catalytic System for N-Arylation of Imidazoles in Water,†Chemistry—A European Journal, 2009, 15, 8971-8974.
(a) R. J. Sundberg, Indoles (Best Synthetic Methods) Academic: London, 1996, 1–6.
(b) A. R. Katritzky, A. F. Pozharskii, Handbook of Heterocyclic Chemistry Pergamon: Oxford, 2000; Chapter 4.
(a) The Alkaloids Specialist Periodical Reports The Chemical Society: London, 1971, 150–200
(b) J. E. Saxton Nat. Prod. Rep. 1989, 6, 1–54
(c) G. A Cordell, Introduction to Alkaloids: A Biogenetic Approach Wiley:New York, 1981, 761–1055
(d) U, Pindur, R.J. Adam Heterocycl. Chem. 1988, 25, 1–8.
(a) N. R. Deprez, D Kalyani, A. Krause, M. S. Sanford, J. Am. Chem. Soc. 2006, 128, 4972–4973;
(b) C. Bressy, D. Alberico, M. Lautens, J. Am. Chem. Soc. 2005, 127,13148–13149.
(c) B. S. Lane, D. Sames, Org. Lett. 2004, 6, 2897–2900;
(d) Toure, B. B.; Lane, B. S. D. J. Sames, Org.Lett. 2006, 8,1979–1982;
(e) B. S. Toure, M. A. Brown, D. J. Sames, Am. Chem. Soc. 2005, 127, 8050–8057.
(a) M. Watanabe, T. Yamamoto, M. Nishiyama, Angew.Chem., Int. Ed. 2000, 39, 2501–2504;
(b) A. Y. Lebedev, V. V. Izmer, D. N. Kazyul, I. P. Beletskaya. A. Z. Voskoboynikov, Org. Lett. 2002, 4,
–626;
(c) M. Watanabe, M. Nishiyama, T. Yamamoto, Y. Koie, TetrahedronLett. 2000, 41, 481–483;
(d) D. W. Old, M. C. Harris, S. L. Buchwald, Org. Lett. 2000, 2, 1403–1406;
(e) K. Kamikawa, S. Kinoshita, H. Matsuzaka, M. Uemura, Org. Lett. 2006, 8, 1097–1100
I. Goldberg, Ber. Deutsch. Chem. Gesell. 1906, 39, 1691–1696.
L. Ackermann, In Modern Arylation Methods; Ackermann, L., Ed.; Wiley-VCH Verlag GmbH, 2009.
F. Ullmann, Ber. Deutsch. Chem. Gesell. 1903, 36, 2382–2384.
B. H. Yang, Buchwald, S. L. J. Organomet. Chem. 1999, 576, 125–146
Kalgauda. B. Gudasi, Siddappa A. Patil, Ramesh S. Vadavi and Rashmi V. Shenoy Transition metal chemistry 2006, 31, 586-582.
V. G. Ricordi, C. S. Freitas, G. Perin, E. J. Lenardão, R. G. Jacob, L. Savegnago, D. Alves, Green Chem. 2012, 14, 1030.
Y. Gu, F. Jérôme, Green Chem. 2010, 12, 1127.
W. Yang, C. Lui, J. Qiu, Chem. Commun. 2010, 46, 2659.
D. H. Lee, M. J. Jin, Org. Lett. 2011, 13, 252.
R. Antony, S. Theodore David, K. Saravanan, K. Karuppasamy, S. Balakumar,
Specrochim. Acta A, 2013, 103, 423.
B. Dai, M. Cao, G. Fang, B. Liu, X. Dong, M. Pan, S. Wang, J. Hazard. Mater, 2012, 103, 219–220
A.A.A. Aziz, A.N.M. Salem, M.A. Sayed, M.M. Aboaly, J. Mol. Struct, 2012, 130, 1010.
ISSN 2348 – 1889
 |
 ISSN 2348 – 1889
ISSN 2348 – 1889