Electrochemistry of Ferrocene in different Room Temperature Ionic Liquids with Platinum and Gold Electrodes
Abstract
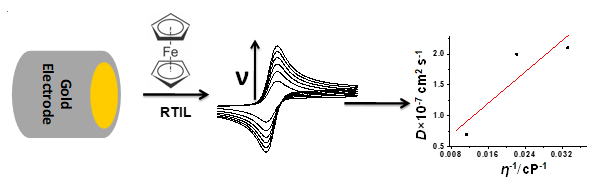
Electrochemical characterization of ferrocene (Fc) was investigated to find out diffusion coefficient (D), heterogeneous rate constant (kº), and relationship between D and kº with Pt and Au electrode in three common room temperature ionic liquids (RTILs) containing namely 1-butyl-1-methyhlpyrrolidinium bis (trifluoromethylsulfonyl) imide, 1-ethyl-3-methylimidazolium trifluoromethylsulfonate and triethylsulfonium bis (trifluoromethylsulfonyl) imide by utilizing cyclic voltammetry and impedance spectroscopy. The D, double layer capacitance (Cdl) and apparent activation energy (ΔGexp) of Fc was deduced in order to elucidate the role of RTIL on the electrode kinetics. The kº of Fc was estimated by electrochemical impedance spectroscopy by using the Randles equivalent circuit as the model. A graph between ln (kº) versus ln (η) suggests that Fc redox process in RTIL with Pt and Au electrodes are adiabatic. The calculated diffusion coefficients and rate constant were of the order of 10−7 cm2 s−1 and 10−2 cm s−1, respectively.
 ISSN 2321-4635
ISSN 2321-4635