Zirconium Antimonoarsanotungstate Zr[SbAsW]: Synthesis and Characterization
Abstract
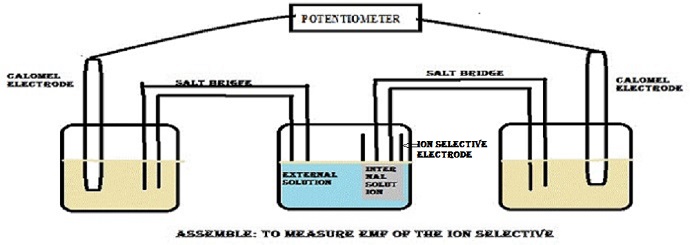
Abstract: In the present research work we concentrated on the structure elucidation of new hetero polyacid salts: Zirconium Antimonoarsanotungstate Zr[SbAsW] salt synthesized at variable pH using sol-gel route. An instrumental technique, FTIR was used to assign the structural aspects to the compound. Physical characterization involves the determination of ion exchange capacity and maximum value for this parameter is 0.40mg/eq. Distribution coefficient values showed that the synthesized compound is preferentially selective for Cu2+ ion. Membrane composition where ZrSbAsW was 40% showed linearity in the range of 1.0x10-4 M to 1.0x10-1 M with a slope of 20.0 mV/decade taking 10-1 M as an internal solution. Above instrumental and analytical studies gave a picture of Cu2+ selective electron-active moiety to the synthesized compound.
Keywords
Full Text:
PDFReferences
C.L. Hill. Introduction: Polyoxometalates Multicomponent Molecular Vehicles To Probe Fundamental Issues and Practical Problems Chem. Rev. 1998, 98, 1-2; and other articles appeared in the issue.
M.T. Pope. Heteropoly and Isopoly Oxometalates, Verlag, Berlin, 1983.
J. F. Keggins. The Structure and Formula of 12-Phosphotungstic Acid Proc. Roy. Soc. A. 1934, 851, 75.
V. Pekarek, V. Vesley. Synthetic inorganic ion exchangers—II:Salts of heteropolyacids, insoluble ferrocyanides, synthetic Aluminosilicates and miscellaneous exchangers. Talanta. 1972, 19, 1245.
Clearfield. Role of ion exchange in solid state chemistry. Chem. Rev. 1988, 88, 125-148.
Clearfield. Inorganic ion exchanger A technology for development Indian. Eng. Chem. Res. 1995, 34, 2865-2872.
W.J. Ball, K.W. Palmer, D.G. Stewart. Process for the production of amorphous aluminosilicates and their use as catalysts, US 4299732 A, 1981.
Lun-Yu, Q.J. Shan, J. Gong, R.Q. Lu, D.R. Wang. Synthesis, properties and characterization of Dawson-type tungstophosphate heteropoly complexes substituted by titanium and peroxotitanium. J. Chem. Soc., Dalton Trans. 1997, 23, 4525-4528.
M. Abe, R. Chitrakar, M. Tsuji, K. Fukumoto. Synthetic inorganic ion-exchange materials XXXIX. Synthesis of titanium(IV) antimonates and their ion exchange properties for alkali and alkaline earth metal ions. Solvent. Extr. Ion Exc. 1985, 3, 149-172.
Z.M. Siddiqi, D. Pathania. Titanium(IV) tungstosilicate and titanium(IV) tungstophosphate: two new inorganic ion exchangers. J. Chromatogr. A. 2003, 987, 147-158.
S.A. Nabi, A.H. Shalla. Synthesis and characterization of a new cation exchanger-zirconium(IV)iodotungstate. J. Por. Mater. 2009, 16, 587-597.
S.A. Khan. Synthesis. Characterization and Ion Exchange Properties of Zirconium (IV) Tungstoiodophosphate, A New Cation Exchanger. Bull. Mat. Sci. 2007, 30(1), 43-49.
S. Siji, C. Janardanan. Studies on Characterization and Catalytic Activities of Bismuth Selective Zirconiumcerium(IV) arsenotungstate Used in Methyl Orange Degradation. Int. J. Adv. Sci. Tech. Res. 2014, 4 (2), 46-55.
Preetha, C. Janardanan, Ion exchange Characteristics of newly Synthesized Cerium Zirconium Phosphotungstate and its Analytical Applications. Res. J. Chem. Sci. 2014, 4(7), 43-51.
H.K. Sharma, N. Sharma. Potentiometric Sensor for Yttrium (III) Based on Tin [IV] Molybdotungtate as an Electroactive Material. Sensor Lett., 2013, 11, 1–5.
C.N.R. Rao. Chemical application of infrared spectroscopy, New York: Academic Press, 1963.
R.P. Buck, R.P. Buck, E. Lindner. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994), Pure Appl. Chem. 1994, 66, 2527-2536.
Y. Umezawa, K. Umezawa, L. Sato. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KA,Bpot values (Technical Report). Pure Appl. Chem. 1995, 67, 507-518
Refbacks
- There are currently no refbacks.
 |
 ISSN: 2456-334X
ISSN: 2456-334X

