
Open Access

Subscription Access
Current status of Regulatory perspectives on Stem Cell Therapy in India
Sandeep Lohmor, Ravinder Verma, Vinay Malik, Deepak Kaushik
Abstract
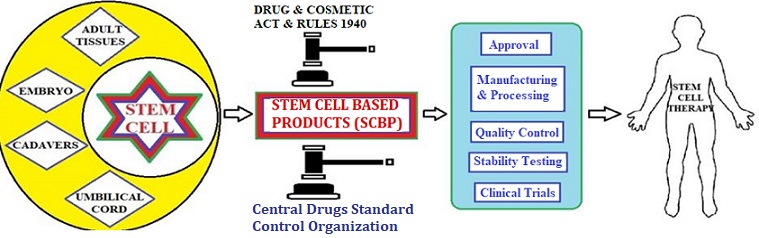
In the recent decade, regenerative medicines are emerging at fast pace in the field of therapeutics. Regenerative medicines have been used in curing severe diseases; mainly through restoration of stem cells. The stem cells are unspecialized cells which have an infinite proliferation capacity and can differentiate into all type of cells. These properties of stem cells to regenerate the functions of the tissues and organs are used in stem cell therapy. The ability of stem cells to regenerate and reconstruct the damaged tissue makes them an exciting area in medical treatment. Stem cell and stem cell-based products are used in the treatment of many diseases and support therapies. In India, the stem cell and cell-based products are supervised by the Central Drug Standard Control Organization (CDSCO) under the Ministry of Health and Family Welfare. CDSCO regulates the clinical trial approvals, monitoring, and approvals of drug manufacturing or new drugs and marketing authorization of stem cell-based products under the Drugs and Cosmetic Act 1940 and Rules. The focus of the present article is to provide information on the procedure of license approval for stem cells and cells based products in India. The article also include discussion on mandatory requirements of stem cell-based products like manufacturing and process controls, quality control, stability testing, labeling, and quality assurance.
Keywords
Stem cell therapy; master cells; stem cell-based products; licensing authority; clinical trials approval;
Full Text:
PDF

ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

