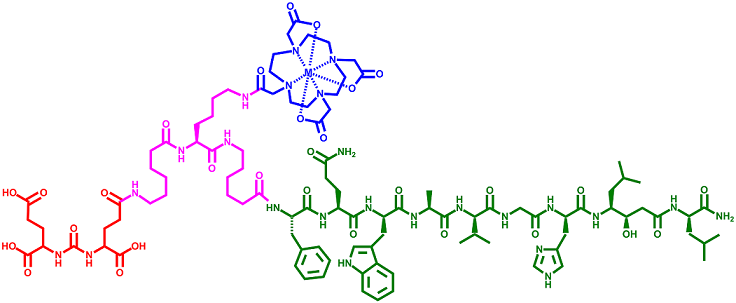
Synthesis and Evaluation of [DUPA-6-Ahx-Lys (DOTA)-6-Ahx-RM2], a Novel, Bivalent Targeting Ligand for GRPr/PSMA Biomarkers of Prostate Cancer
Abstract
In this study, we have prepared a novel, dual-biomarker, targeting ligand having high affinity and specificity for PSMA/GRPr receptors that are expressed on most prostate cancers.  [DUPA-6-Ahx-Lys(DOTA)-6-Ahx-RM2] was synthesized and the new conjugate was metallated macroscopically with GaCl3, InCl3, and LuCl3 to form [DUPA-6-Ahx-Lys(M-DOTA)-6-Ahx-RM2] (where M = Ga, In, or Lu). These new agents, when radiolabeled with Ga-68, In-111, or Lu-177 hold theranostic potential for patients presenting with prostate cancer disease.
Â
Keywords
References
American Cancer Society. Cancer facts & figures, 2017.
S.M. Okarvi. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treatment Reviews. 2008, 34, 13–26.
M. Sutherland, A. Gordon, S. Shnyder, L. Patterson, H. Sheldrake. RGD-binding integrins in prostate cancer: expression patterns and therapeutic prospects against bone metastasis. Cancers. 2012, 4, 1106–45.
A. Katz. Quality of life for men with prostate cancer. Cancer Nurs. 2007, 30, 302–308.
A. Bill-Axelson, L. Holmberg, M. Ruutu, Haggman, S.O. Andersson, S. Bratell, A. Spangberg, C. Busch, S. Nordling, H. Garmo, J. Palmgren, H.O Adami, B.J. Norlen, J.E. Johansson, . Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 2005, 352, 1977–84.
Weber BA, Robert BL, Chumbler NR, Mills TL, Algood CB. Urinary, sexual and bowel dysfunction and bother after radical prostatectomy. Urol. Nurs. 2007, 27, 527–33.
D.J. Brenner, R.E. Curtis, E. Ron. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000, 88, 398–406.
P.S. Birgitte, Jacky, E. Patton, Garay, M. Yanira, F. Joseph, E. Lance, Steward, G. Sanjiv, J. Terrence, Hunt, S. George, R.A. Kei, F.S Ester. Treatments using PSMA ligand endopeptidases. US patent no US20120207742 A1, 2012, Aug 16.
National Cancer Institute. Cancer trends progress report 2009/2010 Update.
A.B. Mariotto, K.R. Yabroff, Y. Shao, E.J. Feuer, M.L. Brown. Projections of the cost of cancer care in the United States: 2010-2020. J. Natl. Cancer Inst. 2011, 103, 117–128.
M.E. Stokes, J. Ishak, I. Proskorovsky, L.K. Black, Y. Huang. Lifetime economic burden of prostate cancer. BMC Health Serv. Res. 2011, 11, 349–54.
D.A. Silver, I. Pellicer, W.R. Fair, W.D. Heston, C. Cordon-Cardo. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85.
S.M. Okarvi. Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Med. Res. Rev. 2004, 24, 357–97.
A. Heppeler, S. Froidevaux, A.N. Eberle, H.R. Maecke. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr. Med. Chem. 2000, 7, 971–94.
A. Varvarigou, P. Bouziotis, C. Zikos, F. Scopinaro, G. De Vincentis. Gastrin-releasing peptide (GRP) analogues for cancer imaging. Cancer Biother. Radiopharm. 2004, 19, 219–29.
R.C. Mease, C.A. Foss, M.G. Pomper. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr. Top Med. Chem. 2013, 13, 951-62.
S.P. Rowe, A. Drzezga, B. Neumaier, M. Dietlein, M.A. Gorin, M.R. Zalutsky, M.G Pomper. Prostate-specific membrane antigen-targeted radiohalogenated PET and therapeutic agents for prostate cancer. J. Nucl. Med. 2016, 57, 90S-96S.
S. Vallabhajosula, I. Kuji, KA. Hamacher, S. Konishi, L. Kostakoqlu, P.A. Kothari, M.I. Milowski, D.M. Nanus, N.H. Bander, S.J. Goldsmith. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J. Nucl. Med. 2005, 46, 634-41.
J.P. Holland, V. Divilov, N.H. Bander, P.M. Smith-Jones, S.M. Larson, J.S. Lewis. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010, 51, 1293-1300.
M. J. Morris, N. Pandit-Taskar, C.R. Divgi, S. Bender, J.A. O’Donoghue, A. Nacca, P. Smith-Jones. L. Schwartz, S. Slovin, R. Finn, S. Larson, H.I. Scher. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin. Cancer Res. 2007, 13, 2707-13.
N. Pandit-Taskar, J.A. O’Donoghue, M.J. Morris, E.A. Wills, L.H. Schwartz, M. Gonen, H.I. Scher, S.M. Larson, C.R. Divgi. Antibody mass escalation study of patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J. Nucl. Med. 2008, 49, 1066-74.
N. Pandit-Taskar, J.A. O’Donoghue, J.C. Durack, S.K. Lyashchenko, S.M. Cheal, V. Beylergil, R.A. Lefkowitz, J.A. Carrasquillo, D.F. Martinez, A.M. Fung, S.B. Solomon, M. Gonen, G. Heller, M. Loda, D.M. Nanus, S.T. Tagawa, J.L. Feldman, J.R. Osborne, J.S. Lewis, V.E. Reuter, W.A. Weber, N.H. Bandar, H.I. Scher, S.M. Larson, M.J. Morris. A Phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin. Cancer Res. 2015, 21, 5277-85.
S.Y. Cho, K.L. Gage, R.C. Mease, S. Senthamizhchelvan, D.P. Holt, A. Jeffrey-Kwanisai, C.J. Endres, R.F,.Dannais, G. Sqouros, M. Lodge, M. A. Eisenberger, R. Rodriquez, M.A. Carducci, C. Rojas, B.S. Sjusher, A.P. Kozikowski, M.G. Pomper. Biodistribution, tumor detection and radiation dosimetry of 18F-DCFBC, a low molecular weight inhibitor of PSMA, in patients with metastatic prostate cancer. J. Nucl. Med. 2012, 53, 1883-91.
A. Afshar-Oromieh, U. Haberkorn, M. Eder, M. Eisenhut, C.M. Zechmann. [68Ga]gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging. 2012, 39, 1085-86.
M. Beheshti, T. Kunit, S. Haim, R. Zakavi, C. Schiller, A. Stephens, L. Dinkelborg, W. Langsteger, W. Loidl. BAY 1075553 PET-CT for staging and restaging prostate cancer patients: comparison with [18F]fluorocholine PET-CT (Phase I study). Mol. Imaging Biol. 2015, 17, 424-33.
X. Chen, R. Park, Y. Hou, M. Tohme, A.H. Shahinian, J.R. Bading, P.S. Conti. MicroPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J. Nucl. Med. 2004, 45, 1390–97.
H. R. Maecke, M. Hofmann, U. Haberkorn. 68Ga-Labeled peptides in tumor imaging. J. Nucl. Med. 2005, 46 (suppl 1), 172S–78S.
W.C. Van de, F. Dumont, B.R. Vanden, W. Oosterlinck, V. Cocquyt, R. Serreyn, S. Peers, J. Thornback, G. Slegers, R.A. Dierckx. Technetium-99 m RP527, a GRP analogue for visualisation of GRP receptor-expressing malignancies: a feasibility study. Eur. J. Nucl. Med. 2000, 27, 1694–99.
H. Zhang, J. Chen, C. Waldherr, K. Hinni, B. Waser, J.C. Reubi, H.R. Maecke. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor-expressing tumors. Cancer Res. 2004, 64, 6707–15.
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24: 389–427.
R. Markwalder, J.C. Reubi. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–59.
J.C. Garrison, T.L. Rold, G.L. Sieckman, S.D. Figueroa, W.A. Volkert, S.S. Jurisson, T.J. Hoffman. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. J. Nucl. Med. 2007, 48, 1327–37.
K.A. Lears, R. Ferdani, K. Liang, A. Zheleznyak, R. Andrews, C.D. Sherman, S. Achilefu, C.J. Anderson, B.E. Rogers. In vitro and in vivo evaluation of 64Cu-labeled SarAr-bombesin analogs in gastrin-releasing peptide receptor-expressing prostate cancer. J. Nucl. Med. 2011, 52, 470–77.
W.A. Breeman, D.M. Jong, J.L. Erion, J.E. Bugai, A. Srinivasan, B.F. Bernard, D.J. Kwekkeboom, T.J. Visser, E.P. Krenning. Preclinical comparison of 111In-labeled DTPA- or DOTA-bombesin analogs for receptor-targeted scintigraphy and radionuclide therapy. J. Nucl. Med. 2002, 43,1650 –56.
A.F. Prasanphanich, P.K. Nanda, T.L. Rold, L. Ma, M.R. Lewis, J.C. Garrison, T.J. Hoffman, G.L.Sieckman, S.D. Fiqueroa, C.J. Smith. [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc. Natl. Acad. Sci USA. 2007, 104, 12462–67.
W.C. Vande, F. Dumont, R. Dierckx, et al. Biodistributaion and dosimetry of 99mTc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor expressing malignancies. J. Nucl. Med. 2001, 42, 1722–27.
R.P. Bandari, Z. Jiang, T.S. Reynolds, N.E. Bernskoetter, A.F. Szczodroski, K.J. Bassuner, D.L. Kirkpatrick, T.L. Rold, G.L.Sieckman, T.J. Hoffman, J.P. Connors, C.J. Smith. Synthesis and biological evaluation of copper-64 radiolabeled [DUPA-6-Ahx-(NODAGA)-5-Ava-BBN(7-14)NH2], a novel bivalent targeting vector having affinity for two distinct biomarkers (PSMA/GRPR) of prostate cancer. Nucl. Med. Biol. 2014, 4, 355–63.
C. Liolios, M. Schäfer, U. Haberkorn, M. Eder, K. Kopka. Novel bispecific PSMA/GRPR targeting radioligands with optimized pharmacokinetics for improved PET imaging of prostate cancer. Bioconjugate Chem. 2016, 27, 737–51.
R. Markwalder, J.C. Reubi. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–59.
M.R. Pillai, R. Nanabala, A. Joy, A. Sasikumar, F.F. Russ Knapp. Radiolabeled enzyme inhibitors and binding agents targeting PSMA Effective theranostic tools for imaging and therapy of prostate cancer. Nuc. Med. Biol. 2016, 43, 692–720.
C.J. Smith, W.A. Volkert, T.J. Hoffman. Gastrin releasing peptide (GRP) receptor targeted radiopharmaceuticals: a concise update. Nucl. Med. Biol. 2003, 30, 861–8.
J.C. Reubi. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427.
B.A. Nock, A. Nikolopoulou, A. Galanis, P. Cordopatis, B. Waser, J.C. Reubi, T. Maina. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: a preclinical study. J. Med. Chem. 2005, 48, 100–10.
J.J. Parry, T.S. Kelly, R. Andrews, B.E. Rogers. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin(7-14) analogues containing different amino acid linker moieties. Bioconjug. Chem. 2007, 18, 1110–17.
C.J. Smith, L.S. Gary, K.O. Nellie, L.H. Donald, G.M. Dana, K. Raghuraman, A.V. Wynn, T.J. Hoffman. Radiochemical Investigations of Gastrin-releasing Peptide Receptor-specific [99mTc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, Tumor-bearing, Rodent Models: Syntheses, Radiolabeling, and in Vitro/in Vivo Studies where Dpr = 2,3-Diaminopropionic acid and X = H2O or P(CH2OH)3. Cancer Res. 2003, 63, 4082– 88.
Y.S. Yang, X. Zhang, Z. Xiong, X. Chen. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogues in a mouse model of human prostate adenocarcinoma. Nucl. Med. Biol. 2006, 33, 371– 80.
E. Gourni, P. Bouziotis, D. Benaki, G. Loudos, S. Xanthopoulos, M. Paravatou-Petsotas, M. Marvi-vavagianni, M. Pelecanou, S.C. Archimandritis, A.D. Varvarigou. Structural assessment and biological evaluation of two N3S bombesin derivatives. J. Med. Chem. 2009, 52, 4234 – 46.
F. Scopinaro, G. Vincentis, A. D. Varvarigou, C. Laurenti, F. Iori, S. Remediani, S. Chiarini, S. Stella. 99mTc-bombesin detects prostate cancer and invasion of pelvic lymph nodes. Eur. J. Nucl. Med. Mol. Imaging. 2003, 30, 1378–82.
K. Durkan, Z. Jiang, T.L. Rold, G.L. Sieckman, T.J. Hoffman, R.P. Bandari, A.F. Szczodroski, L. Liu, Y. Miao, T.S. Reynolds, C.J. Smith. A heterodimeric [RGD-Glu-[64Cu-NO2A]-6-Ahx-RM2] αvβ3/GRPr-targeting antagonist radiotracer for PET imaging of prostate tumors. Nucl. Med. Biol. 2014, 41, 133–9.
R. Minamimoto, S. Hancock, B. Schneider, F.T. Chin, M. Jamali, A. Loening, S. Vasanawala, S.S. Gambhir, A. Iagaru. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2016, 57, 557-62.
G. Wieser, R. Mansi, A.L. Grosu, W. Schultze-Seemann, R.A. Dumont-Walter, P.T. Meyer, H.R. Maecke, J.C. Reubi, W.A. Weber. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist from mice to men. Theranostics. 2014, 4, 412-19.
T. Maina, H. Bergsma, H.R. Kulkarni, D. Mueller, D. Charalambidis, E.P. Krenning, B.A. Nock, M. de Jong, R.P. Baum. Preclinical and first clinical experience with the gastrin-releasing peptide receptor antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2016, 43, 964-73.
Z. Liu, Z.B. Li, Q. Cao, S. Liu, F. Wang, X. Chen. Small-animal PET of tumors with 64Cu-labeled RGD-bombesin heterodimer. J. Nucl. Med. 2009, 50, 1168–77.
Z. Liu, G. Niu, F. Wang, X. Chen. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging. 2009, 36, 1483–94.
Z. Liu, Y. Yan, F.T. Chin, F. Wang, X. Chen. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using18F-labeled PEGylated RGD bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J. Med. Chem. 2009, 52, 425–32.
M. Eder, M. Schafer, U. Bauder-Wust, U. Haberkorn, M. Eisenhut, K. Kopka. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate. 2014, 74, 659−68.
A.P. Kozikowski, J. Zhang, F. Nan, P.A. Petukhov, E. Grajkowska, J.T. Wroblewski, T. Yamamoto, T. Bzdega, B. Wroblewska, J.H. Neale. . Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J. Med. Chem. 2004, 47, 1729–38.
K. Graham, R. Lesche, A.V. Gromov, N. Bohnke, M. Schafer, J. Hassfeld, L. Dinkelborg, G. Kettaschau. Radio fluorinated derivatives of 2-(phosphonomethyl)pentanedioic acid as inhibitors of prostate specific membrane antigen (PSMA) for the imaging of prostate cancer. J. Med. Chem. 2012, 55, 9510–20.
A.J. Vander-Lely, W.W. Herder, E.P. Krenning, D.J. Kwekkeboom. Octreoscan radioreceptor imaging. Endocrine. 2003, 20, 307–11.
R. A. Dumont, M. Tamma, F. Braun, S. Borkowski, J.C. Reubi, H. Maecke, W.A. Weber, R. Mansi. Targeted radiotherapy of prostate cancer with a gastrin-releasing peptide receptor antagonist is effective as monotherapy and in combination with rapamycin. J. Nucl. Med. 2013, 54, 762–69.
F. Hu, C.S. Cutler, T. Hoffman, G. Sieckman, W.A. Volkert, S.S. Jurisson. Pm-149 DOTA bombesin analogs for potential radiotherapy in vivo comparison with Sm-153 and Lu-177 labeled DO3A-amide-betaAla-BBN(7-14)NH(2). Nucl. Med. Biol. 2002, 29, 423–30.
C.V. Johnson, T. Shelton, C.J. Smith, L. Ma, M.C. Perry, W.A. Volkert, T.J. Hoffman. Evaluation of combined [177Lu-DOTA-8-AOC-BBN (7-14)NH2] GRP receptor-targeted radiotherapy and chemotherapy in PC-3 human prostate tumor cell xenografted SCID mice. Cancer Biother. Radiopharm. 2006, 21, 155–66.
L.E. Lantry, E. Cappelletti, M.E. Maddalena, J.S. Fox, W. Feng, J. Chen, R. Thomas, S.M. Eaton, N.J. Bogdan, T. Arunachalam, J.C. Reubi, N. Raju, E.C. Metcalfe, L. Lattuada, K.E. linder, R.E. Swenson, M.E. Tweedle, A.D. Nunn 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J. Nucl. Med. 2006, 47, 1144–52.
C.J. Smith, H. Gali, G.L. Sieckman, D.L. Hayes, N.K. Owen, D.G. Mazuru, W.A. Volkert, T.J. Hoffman. Radiochemical investigations of 177Lu-DOTA-8-Aoc-BBN[7-14]NH2: an in vitro/in vivo assessment of the targeting ability of this new radiopharmaceutical for PC-3 human prostate cancer cells. Nucl. Med. Biol. 2003, 30, 101–09.
H. Zhang, J. Chen, C. Waldherr, K. Hinni, B. Waser, J.C. Reubi, H.R. Maecke. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with Indium-111, Lutetium-177, and Yttrium-90 for targeting bombesin receptor expressing tumors. Cancer Res. 2004, 64, 6707–15.
E. Koumarianou, R. Mikolajczak, D. Pawlak, X. Zikos, P. Bouziotis, P. Garnuszek, U. Karczmarczyk, M. Maurin, S.C. Archimandritis. Comparative study on DOTA-derivatized bombesin analog labeled with 90Y and 177Lu: In vitro and in vivo evaluation. Nucl. Med. Biol. 2009, 36, 591–603.
S. Dapp, C. Muller, E.G. Garayoa, P. Blauenstein, V. Maes, L. Brans, D.A. Tourwe, R. Schibli. PEGylation, increasing specific activity and multiple dosing as strategies to improve the risk benefit profile of targeted radionuclide therapy with 177Lu-DOTA-bombesin analogues. EJNMMI Res. 2012, 2, 24.
H. Zhang, J. Schuhmacher, B. Waser, D. Wild, M. Eisenhut, J.C. Reubi, H.R. Maecke. DOTA-PESIN, a DOTA-conjugated bombesin derivative designed for the imaging and targeted radionuclide treatment of bombesin receptor-positive tumors. Eur. J. Nucl. Med. Mol. Imaging. 2007, 34,1198–1208.
L. Jiang, Z. Miao, H. Liu, G. Ren, A. Bao, C.S. Cutler, H.Shi, Z. Cheng. 177Lu-labeled RGD-BBN heterodimeric peptide for targeting prostate carcinoma. Nucl Med Commun. 2013, 34, 909–14.
W.P. Li, C.J. Smith, C.S. Cutler, T.J. Hoffman, A.R. Ketring, S.S. Jurisson. Aminocarboxylate complexes and octreotide complexes with no carrier added 177Lu, 166Ho and 149Pm. Nucl. Med. Biol. 2003, 30, 241–51.
C.S. Cutler, M. Wuest, C.J. Anderson, D.E. Reichert, Y. Sun, A.E. Martell, M.J. Welch. Labeling and in vivo evaluation of novel copper(II) dioxotetraazamacrocyclic complexes. Nucl. Med. Biol. 2000, 27, 375–80.
C.S. Cutler, C.J. Smith, G.J. Ehrhardt, T.T. Tyler, S.S. Jurisson, E. Deutsch. Current and potential therapeutic uses of lanthanide radioisotopes. Cancer Biother. Radiopharm. 2000, 15, 531–45.
M.F. Bin-Othman, R. Nabil, A. Mitry, J. Valerie, B. Lewington, P.J. Blower, Y.A. Samantha. Re-assessing gallium-67 as a therapeutic radionuclide. Nuc. Med. Biol. 2016, 46, 12–18.
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

