Role of low salt concentration on electrical conductivity in blend polymeric films
Abstract
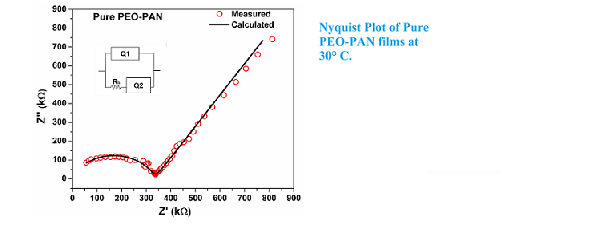
A new blend polymer electrolyte based on polyethyelene oxide (PEO) and polyacrylonitrile (PAN) doped with Lithium Hexafluorophosphate (LiPF6) has been prepared by solution casting technique using Dimethyalformamide (DMF) as solvent. The prepared samples were characterized by FTIR, FESEM and ac impedance spectroscopic measurements. The complex formation between blend polymer (0.5g PEO: 0.5g PAN) and LiPF6 has been studied using Fourier transform infrared spectroscopy (FTIR). From AC impedance spectroscopic analysis there is enhancement of two order on addition of salt than pure PEO-PAN. The effect of low salt concentration on the conductivity and surface morphology of the blend polymer electrolyte has been discussed.
Keywords
electrical; FTIR; FESEM; polymer films; impedance spectroscopy
 ISSN 2321-4635
ISSN 2321-4635