Synthesis, DNA photocleavage, molecular docking and anticancer studies of 2-methyl-1,2,3,4-tetrahydroquinolines
Abstract
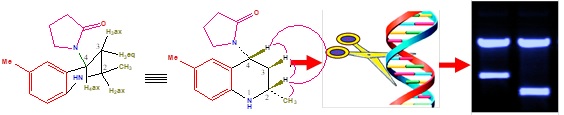
2-Methyl-1,2,3,4-tetrahydroquinolin-4-yl)pyrrolidin-2-ones (3a−g) were synthesized by one pot multicomponent aza Diels-alder reaction between N-arylimines with two molecules of N-vinyl-2-pyrrolidinone in presence of Sm(III)nitrate as catalyst in acetonitrile solvent at room temperature stirring. The photocleavage studies with 2-methyl-1,2,3,4-tetrahydroquinolin-4-yl)pyrrolidin-2-ones (3a−g) revealed that almost all derivatives exhibited effective photocleavage of pUC−19 DNA at 365 nm, The The anticancer activities of newly synthesized compounds (3a−g) were more potent than doxorubicin on MCF−7 cells. The docking of PBR receptor (1EQ1) protein with newly synthesized THQ’s (3a-h) exhibited well established bonds with one or more amino acids in the receptor active pocket.
Keywords
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

