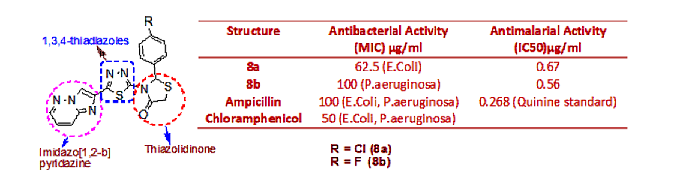
Synthesis, antimicrobial and antimalarial study of novel 1,3,4-thiadiazole derivatives incorporating imidazo [1,2-b] pyridazine and thiazolidinone moieties
Abstract
Keywords
References
S. Caravita, A. Faini, G. Bilo, M. Lang, G. Parati. Role of acetazolamide and telmisartan/nifedipine-GITS combination in antagonizing the blood pressure rise induced by high altitude exposure. Int. J. Cardiol., 2016, 225, 324-326.
S. Fossati, P. Giannoni, M.E. Solesio, S.L. Cocklin et al., The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol. Dis., 2016, 86, 29-40.
C. Boda, B. Enanga, H. Dumet, G. Chauviere, F. Labrousse et al,. Plasma kinetics and efficacy of oral megazol treatment in Trypanosoma brucei brucei-infected sheep. Vet. Parasitol., 2004, 121(3), 213-223.
K. Kusakabe, N. Ide, Y. Daigo, T. Itoh, T. Yamamoto et al. Discovery of Imidazo[1,2-b]pyridazine Derivatives: Selective and Orally Available Mps1 (TTK) Kinase Inhibitors Exhibiting Remarkable Antiproliferative Activity. J. Med. Chem., 2015, 58(4), 1760–1775.
S. Moreau, P. Coudert, C. Rubat, D. Vallee-Goyet et al. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg. Med. Chem., 6(7), 1998, 983-991.
A. Bhatt, R.K. Singh, R. Kant. Synthesis of novel Imidazo [1,2-b] pyridazine derivatives and study of their biomedicinal efficacy. Chem. Biol. Lett., 2016, 3(2), 38-43.
H. Chen, A. Rusinko, M.R. Hellberg, B.S. Severns, A.J. Henderson et al., 6-aminoimidazo[1,2-b]pyridazine analogs as rho kinase inhibitors for the treatment of rho kinase-mediated diseases and conditions. US 2008/0153813 A1.
J. Singh, B.S. Chhikara. Comparative global epidemiology of HIV infections and status of current progress in treatment. Chem. Biol. Lett., 2014, 1(1), 14-32.
J. Knight, I. David, I. Scopes, C. David. Imidazopyridayine derivatives having antiviral activity. US Patent 1987/4690917.
M. GraziaRimoli, E. Russo, M. Cataldi, R. Citraro, P. Ambrosino. T-type channel blocking properties and antiabsence activity of two imidazo[1,2-b]pyridazine derivatives structurally related to indomethacin. Neuropharmacol., 2009, 56(3), 637-646.
E. Palazzo, M. GraziaRimoli, M. De Chiaro, F. Guida, D. Melisi et al., Intra-periaqueductal grey microinjections of an imidazo[1,2-b]pyridazine derivative, DM2, affects rostral ventromedial medulla cell activity and shows antinociceptive effect. Neuropharmacol., 2010, 58(3), 660-667.
P. Borowski, M. Lang, A. Haag, H. Schmitz. Characterization of Imidazo[4,5-d]Pyridazine Nucleosides as Modulators of Unwinding Reaction Mediated by West Nile Virus Nucleoside Triphosphatase/Helicase: Evidence for Activity on the Level of Substrate and/or Enzyme. Antimicrob. Agents Chemother. 2002, 46(5), 1231-1239.
R. Singh, T. Arif, I. Khan, P. Sharma. Phytochemicals in antidiabetic drug discovery. J. Biomed. Therapeutic Sci., 2014, 1(1), 1-33.
J. Blanchet, J. Zhu. Reeve's synthesis of 2-imino-4-thiazolidinone from alkyl (aryl) trichloromethylcarbinol revisited, a three-component process from aldehyde, chloroform and thiourea, Tetrahedron Lett., 2004, 45, 4449-4452.
R. Ottana, R. Maccari, M.L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, D.R. Paola, L. Sautebin, S. Cuzzocrea, M.G. Vigorita. 5-Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents. Biorg. Med. Chem.2005, 13, 4243-4252.
K.A. Kandeel. Synthesis and structures of some new thiazolidin-4-ones and thiazolin-4-ones of anticipated biological activity, Arkivoc, 2006, 10, 1-6.
D.S. Mehta, V.H. Shah. Synthesis and biological Activity of 2- azetidinones,4- thiazolidinones,5- imidazolinones having benzthiazole moiety. Indian J Heterocycl Chem. 2001, 11, 139-144.
S.G. Kucukguzel, E.E. Orul, S. Rollas, F. Salin, A. Ozbek. Synthesis, characterization and biological activity of novel 4-thiazolidinones, 1, 3, 4-oxadiazoles and some related compounds. Eur. J. Med. Chem, 2002, 37, 197-206.
R.K. Rawal, Y.S. Prabhakar, S.B. Katti E. DeClercq. 2-(Aryl)-3-furan-2-ylmethylthiazolidin-4-ones as selective HIV-RT Inhibitors. Bioorg. Med. Chem, 2005, 13, 6771- 6776.
J.J. Bronson, K.L. DenBleyker, P.J. Falk, R.A. Mate, H.T. Ho, M.J. Pucci, L.B. Snyder. Discovery of the first antibacterial small molecule inhibitors of Mur B. Bioorg. Med. Chem. Lett., 2003, 13, 873.
W. Cunico, C.R.B. Gomes, W.T. Vellasco Jr.. Chemistry and Biological Activities of 1,3-Thiazolidin-4-ones. Mini Rev. Org. Chem., 2008, 5(9), 336-344.
A. Verma, S.K. Saraf. 4-Thiazolidinone – A biologically, active scaffold. Eur. J. Med. Chem., 2008, 43, 897-905.
W.S. Hamama, M.A. Ismail, S. Shaaban, H.H. Zoorob. Progress in the chemistry of 4- thiazolidinones. J. Heterocyclic Chem., 2008, 45(4), 939-956.
H.D. Isenberg, Clinical microbiology procedure handbook, vol II, 2nd edition, chapter 5, 1999, p. 501.
N.C. Desai, P.N. Shihora, D.L. Moradia. Synthesis and characterization of new quinazolines as potential antimicrobial agents. Indian J. Chem., 2007, 46B, 550-553.
S. Shadomy. In Manual of Clinical Microbiology; Albert,B., Ed.; ASM Press: Washington, DC, 1999; p 1173.
A. Rattan, Antimicrobials in Laboratory Medicine; BIChurchill Livingstone: India, 2000; p 85.
ISSN 2347–9825
Authors/visitors are advised to use Firefox browser for better experience of journal site.
Open Access: Researcher from developing/low economy countries can access the jorunal contents through WHO-HINARI .
 ISSN 2347-9825
ISSN 2347-9825

